It is no secret that the world is facing an energy crisis. Global demand for fossil fuels continues to rise due to the growth of economies in Asia, South America and Sub-Saharan Africa. Meanwhile, analysts predict that "peak oil" production has already come and gone, while others expect production rates to decline as soon as 2020. At the same time, the environmental impact of increasing carbon emissions is already being felt.
According to the National Oceanic and Atmospheric Administration (NOAA), carbon dioxide concentrations in the upper atmosphere reached 400 parts per million (ppm) last year. And by 2100, depending on rates of consumption, carbon dioxide emissions could either level off at about 550 ppm or rise to as high as 800. This could mean the difference between a temperature increase of 2.5 degrees Celsius (4.5 °F), which is sustainable, and 4.5 °C (8 °F), which is not!
It is little wonder then why scientists are increasingly looking to nature for possible solutions to our energy problems. Beyond the promise of alternative energy sources - i.e. solar, wind, tidal,and geothermal - there is also considerable work being done to harness alternative fuels. And methods that can generate alternative fuels in ways that are environmentally-friendly and cheap are especially worthwhile.
That's where "artificial leaf" technology comes into play, a green approach to making hydrogen fuel that copies plants' ability to convert sunlight into hydrogen fuel. And now, a team of researchers, with funding from the U.S. Department of Energy and the Singapore-Berkeley Research Initiative for Sustainable Energy, have reported making progress towards a stand-alone system that lends itself to large-scale, low-cost production.
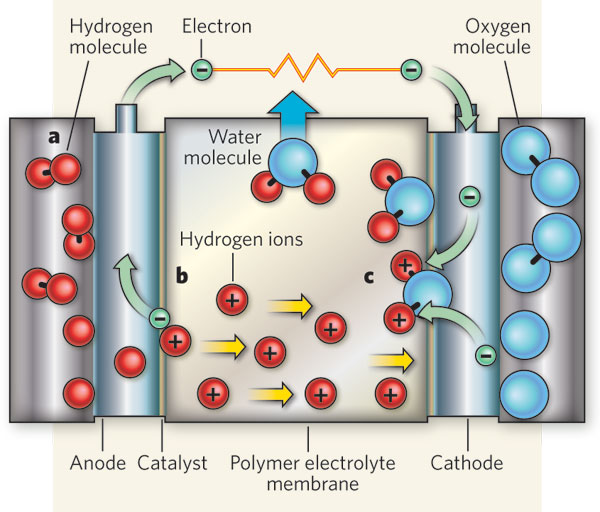
For years, scientists have been pursuing the concept, otherwise known as a photoelectrochemical (PEC) cell technology. In contrast to a photovoltaic cell (i.e a solar cell, which converts light to electricity), a PEC cell collects photons and converts them to energetic electrons that touch off a chemical reaction that splits water into hydrogen and oxygen gas. These gases can then be turned into power by being placed into a hydrogen fuel cell (or "Bacon Cell"), which generates electricity by recombining these gases.
Peidong Yang, Bin Liu, and the researchers responsible for this latest development describe their nanowire mesh design in the journal ACS Nano under the title "All Inorganic Semiconductor Nanowire Mesh for Direct Solar Water Splitting". In their paper, they note that harnessing sunlight to split water and harvest hydrogen is one of the most intriguing ways to achieve clean energy.
Automakers have started introducing hydrogen fuel cell vehicles, which only emit water when driven. But making hydrogen, which mostly comes from natural gas, requires electricity from conventional carbon dioxide-emitting power plants.
Producing hydrogen at low cost from water using the clean energy from the sun would make this form of energy, which could also power homes and businesses, far more environmentally friendly. Building on a decade of work in this area, Yang's team has achieved another important step toward this goal.
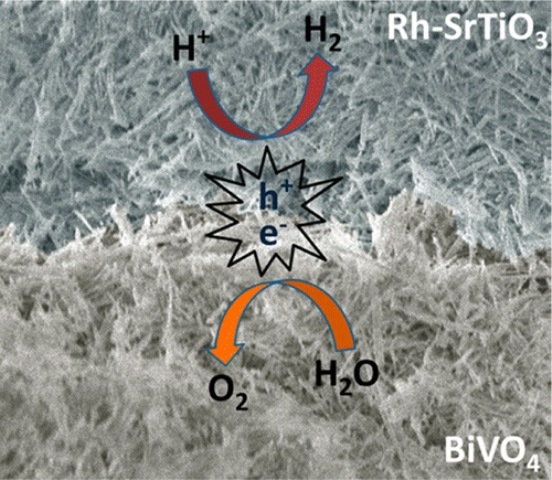
Taking a page from the paper industry, they produced a sheet-like flat mesh out of light-absorbing semiconductor nanowires that, when immersed in water and exposed to sunlight, produces hydrogen gas. The scientists say that the technique could allow their technology to be scaled up at low cost.
Although boosting efficiency remains a challenge, their approach - unlike other artificial leaf systems - is free-standing and doesn't require any additional wires or other external devices that would add to the environmental footprint.
And speaking of efficiency, in May of last year, researchers from the National Institute of Standards and Technology (NIST) produced a study that proposed creating a new, more efficient PEC. This design would consist of a layer of silicon dioxide to insulate the semiconductor, while a top layer would consist of an array of tiny electrodes made of titanium and coated with platinum.
As described by Yang and Liu, the protective layer of silicon dioxide is thin enough to admit a sufficient amount of light. "[P]hotons will travel through it to the semiconductor," they claim, "and the photo-generated electrons will 'tunnel' in the opposite direction to reach the electrodes, where the platinum catalyzes the reaction that produces hydrogen.”
The NIST study also notes that part of the savings would come in during the manufacturing process, which could be based on standard fabrication methods that are already common throughout the electronics industry.
Similarly, for years now, researchers led by MIT Professor Daniel Nocera have been pursuing artificial leaf technology. After years of work, they produced an artificial leaf that consists of a thin layer of silicon - what most solar cells are made from - that is coated with a cobalt-based catalyst on one side (which released oxygen from wate molecules), and a layer of a nickel-molybdenum-zinc alloy on the other - which releases hydrogen.
Much like Yang and Liu's concept, Nocera's "leaf" needs no external wires or control circuits to operate and quickly begins to work the moment it's added to water. Simply placed in a container of water and exposed to sunlight, it quickly begins to generate streams of bubbles: oxygen bubbles from one side and hydrogen bubbles from the other.
If placed in a container that has a barrier to separate the two sides, the two streams of bubbles can be collected and stored, and used later in a hydrogen fuel cell to generate electrical power.
The creation of the device is described in a paper published in Sept. 30th, 2011 in the journal Science. The device, Nocera explains, is made entirely of earth-abundant, inexpensive materials — mostly silicon, cobalt and nickel — and works in ordinary water. Other attempts to produce devices that could use sunlight to split water have relied on corrosive solutions or on relatively rare and expensive materials such as platinum.
“I think there’s going to be real opportunities for this idea,” Nocera said. “You can’t get more portable - you don’t need wires, it’s lightweight... You just drop it in a glass of water, and it starts splitting it."
While significant hurdles still need to be overcome before this sort of technology can become mainstream, these and other developments are all very encouraging. In addition to offering alternatives, concepts like the artificial leaf are designed with both sustainability and cost-effectiveness in mind.
This combined approach of addressing future needs in ways that are clean and inexpensive are the very hallmarks of alternative energy and fuel research. As it stands, every predictive scenario we have today indicates that demands for energy will rise while resources simultaneously dwindle, due to increased consumption and the effects of Climate Change. As such, relying on conentional means of energy-production (i,e coal, oil and natural gas) simply will not cut it.
But as the saying goes, necessity is the mother of invention, and there are few things more necessary than making sure that future generations have what they need to live, strive and survive.
And be sure to check out this video from the Nocera Lab and Sun Catalytix that shows the "artificial leaf" in action:
Sources:
- www.eurekalert.org/pub_releases/2014-12/acs-tal120314.php
- newsoffice.mit.edu/2011/artificial-leaf-0930
- pubs.acs.org/doi/abs/10.1021/nn5051954
- svs.gsfc.nasa.gov/cgi-bin/details.cgi?aid=11280
- www.nist.gov/public_affairs/tech-beat/tb20130514.cfm
- earthobservatory.nasa.gov/Features/GlobalWarming/page5.php
- www.climate.gov/news-features/understanding-climate/2013-state-climate-carbon-dioxide-tops-400-ppm
- www.acs.org/content/acs/en/pressroom/presspacs/2014/acs-presspac-december-3-2014/toward-a-low-cost-artificial-leaf-that-produces-clean-hydrogen-fuel.html
Image Credits: