In honor of the 2015 World Cancer Day, on February 4, the Bonnie J. Addario Lung Cancer Foundation and Free To Breathe are collaboratively launching a unique crowdsourcing challenge, the Clinical Trial Innovation Prize.
The Clinical Trial Innovation Prize is a unique two-part challenge that will award $75,000 USD to identify innovative ways to increase the number of patients participating in cancer clinical trials.
-
Ideation Challenge: Send in creative and novel ideas on how to double the accrual rate of cancer clinical trials, and win prizes totaling $30,000 USD. Register now, and submit your innovative ideas!
-
Implementation/ Proof of Concept Challenge: Win prizes totaling $45,000 USD when you provide proof that your ideas have indeed resulted in an increase in participation in cancer clinical trials. The Implementation Challenge will go live in early February 2016. Competitors are not required to participate in the Ideation Challenge to enter the Implementation Challenge, however, we highly encourage you to do so!
The Problem
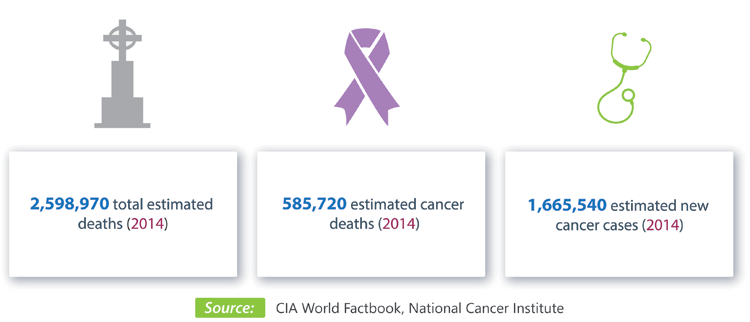
Cancer is one of the leading causes of death worldwide, with the number of new cases expected to rise by approximately 70% over the next two decades.
We need better ways to prevent, detect, screen and treat cancer patients, and we need them now!
The most reliable and the only accepted scientific method to take discoveries from the lab bench to the bedside is testing the safety and efficacy of these new interventions in clinical trials -- research studies that test new methods of prevention, detection, screening and treatment of cancer.
The U.S. National Institutes of Health database currently lists over 45,000 cancer-related clinical trials worldwide.
Unfortunately, more than 20% of these trials will never complete, for reasons unrelated to the effectiveness of the intervention that's being tested! That is a huge drain on the already very limited research $$ and resources, will delay clinical research and getting useful diagnostics and treatments to cancer patients.
The most common reason for 20% of all clinical trials never finishing is poor patient accrual (the process of recruiting patients to the trial for participation), i.e. not enough patients volunteered to join the clinical trial.
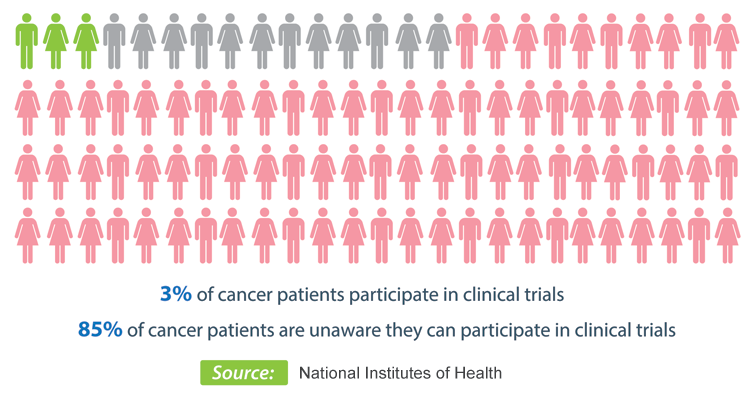
On average, less than 3% of all cancer patients participate in clinical trials!
Participation is even lower among particular groups, including people who are racial and ethnic minorities, over 65, lower income, and living in rural areas. As an example, ~53% of new cancer diagnoses are in people 65 or older, however, this age demographic accounts for a measly 33% of clinical trial participants
Some of the most common reasons for poor patient accrual to cancer clinical trials include:
- a lack of community support,
- patient awareness- As an example of the steep hill that we need to climb, 85% of cancer patients aren't even aware that their treatment facility conducts clinical trials in which they might be eligible to participate.
- Physician awareness,
- stigmas and misconceptions,
- fear of financial or time burden, and
- procedural inefficiencies (such as complexities in enrolling in the trial itself, the informed consent process,), among numerous others.
Why an Innovation Prize?
We know that amazing ideas can come from anywhere. Despite 50 years of trying, members of the medical community have been unable to develop transformational solutions to these intractable problems.
So, we are trying something different! We want you to help us find cool ways to double the accrual rate of cancer clinical trials!
We believe an Innovation Prize could have a profound impact on improving cancer care, too. Help us make it happen!
The Challenge Breakthrough
The Clinical Trial Innovation Prize is looking for innovative means to double the number of cancer patients participating in clinical trials, increasing the accrual rate for cancer clinical trials from 1-3% to 3-6%.
Increased participation in clinical trials will hopefully accelerate the pace of research and drug development allowing patients to live longer and better lives.
Prizes
The Clinical Trial Innovation Prize will award $75,000 USD in total prizes.
The Judging Panel will select the first and second prize winners for the Ideation Challenge. Our judges will also select a group of finalists to be eligible for the People's Choice Award, the winner of which will be selected by a public vote.
The finalist receiving the most votes will be awarded the People's Choice Award. A competitor is eligible to win both a Judges' Award and the People's Choice Award. All votes will be monitored and any indication of impropriety will result in disqualification from the challenge.
Ideation Challenge Winners:
- First Prize: $20,000
- Second Prize: $5,000
- People's Choice Award: $5,000
Implementation Challenge Winner: $45,000
Timeline
| Date |
Challenge Phase |
| February 4, 2015 |
Challenge launches
Registration opens
|
| May 6, 2015 |
Original Deadline for submissions (No longer applicable) |
| May 18, 2015 |
NEW DEADLINE FOR SUBMISSIONS (9pm EDT). Deadline extended!! |
| July 10, 2015 |
Judging Panel Reviews complete |
| July 16, 2015 |
Top Finalists announced for voting |
| August 3, 2015 |
Voting closes |
| August 10 2015 |
Ideation Challenge Winners announced |
| February 2016 |
Implementation Challenge Launches |
Submission
All Ideation Challenge submissions will be made public upon submission to allow for an exchange of ideas, as well as allowing innovators to fine-tune their submissions based on feedback they get from the crowd.
You can make changes to your entry until the final submission deadline on May 18, 2015, 9pm ET. No changes can be made to submissions after this time.
|
What to Create for the Ideation Challenge?
Fill out the submission form with a catchy title, a brief 140 character description, an image that illustrates your submission or amplifies your message, and a 2 page description as detailed on the submission page addressing specific questions below around the challenge (space limitations are included in the submission form). The 2 page description should be no less than 11pt font with 1 in margins.
|
|
Barriers
- What barriers are you addressing?
- Why do these barriers exist?
- Why are you addressing these barriers?
- How/why do the barriers prevent cancer patients from participating in clinical trials?
- Cite 3 sources of information that illustrate/describe the problem.
|
|
Proposed Solution
What is your proposed solution to addressing the barriers?
- Description
- How will you do it?
- How does it work?
- What does it look like?
- How will you implement it?
- Where will you implement?
- Who will be involved (stakeholders)?
- How much will it cost to create the solution (an estimation)?
- How much will it cost to implement the solution (an estimation)?
- How many people will be impacted?
- How long will it take to create the proposed solution?
- Why will it work? Why is it viable?
|
|
Potential Obstacle(s)
- What are potential obstacles to your solution?
- How will you work around these roadblocks?
|
|
Potential Impact
- What % increase in accrual rates do you anticipate?
- Why?
- How will you prove it?
- How long will it take to achieve this increase with your solution?
|
|
Sustainability of Solution
- How do you anticipate maintaining the % increase in accrual rates over time?
|
|
Regulatory Hurdles
- How will you overcome legal/regulatory hurdles, if any?
|
|
Proposed Innovation
- Why hasn’t your proposed solution been tried before?
- If it has, what prevented it from succeeding?
|
Judging Panel
The Judging Panel comprises of thought leaders in the business, academic and non profit worlds. Judges will evaluate all submissions on compliance with the rules and guidelines of the Prize, and will have the sole and absolute discretion to select the winners. The Judging Panel’s decisions are final, binding and are not subject to challenge.
All Judges will be required to sign and adhere to non-disclosure agreements as required and statements acknowledging that they make no claim to the Intellectual Property developed by teams.
Judging Criteria
All submissions will be evaluated on the following weighted criteria:
| Criteria |
Score |
| Solution Design |
20 points |
| Innovation of Solution |
20 points |
| Viability of Solution |
20 points |
|
Potential of sustainability and
scalability of solution
|
15 points |
| Implementation Plan |
10 points |
| Problem analysis and scope of solution |
10 points |
| Cost |
5 points |
TOTAL: 100 points
Challenge Details
Rules
Challenge rules are subject to change. All registered teams and individuals will be informed of any rule changes and any changes will be posted on the Challenge page.
Submissions
All Ideation Challenge submissions will be made public upon submission to allow for an exchange of ideas, as well as allowing innovators to fine-tune their submissions based on feedback they get from the crowd.
You can make changes to your entry until the final submission deadline on May 18, 2015, 9pm ET. No changes can be made to submissions after this time.
Your challenge submission remains your own. By participating in this challenge, each competitor agrees to submit only their own idea. Any indication of "copying" another competitors' idea is grounds for disqualification from the challenge.
People's Choice Award selected by a Public Vote
The Judging Panel will select a group of finalists to be eligible for the People's Choice Award. The finalist receiving the most votes will be awarded the People's Choice Award. A competitor is eligible to win both a Judges' Award and the People's Choice Award. All votes will be monitored and any indication of impropriety will result in disqualification from the challenge.
Contact Us:
Have questions? Send us an email at clinicaltrialprize@lungcancerfoundation.org, and we'll get back to you rightaway!










