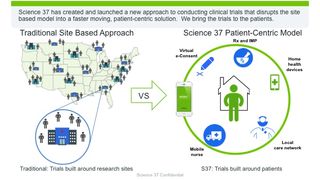
Our model alleviates the enrollment barriers surrounding patient access, awareness, geography and associated travel constraints, and trust.
Most patients do not live in close proximity to a research site. Patients that live near a site may not feel well enough to travel to the site or may be concerned about taking time out of their day. Patient awareness and mistrust of the medical community and the research field itself may also prevent patients from enrolling (see source links).
Science 37 believes that if you restructure research to make it more responsive to the needs and concerns of patients, more patients will participate.
One potential obstacle is that patients may not have access to proper technology (i.e. smartphone/tablet, internet access). Another is that the patient may feel a lack of connection to the physician-investigator.
Patients enrolled in clinical trials will be provided with the necessary technology to be used from the comfort of their home or in research settings. To prevent patients from feeling a lack of connection to the physician-investigator, patients will have real-time access through telephone and video calls in NORA to trial staff.
We anticipate that clinical trial accrual rates will at least double using the Science 37 approach. Through our robust digital recruitment and patient-centric strategies, we will target appropriate patient audiences regardless of location, increase awareness, and ensure clinical trial integrity, all while maintaining patient trust and safety. Accrual rates will be proven upon completion of a successful clinical trial. Our management system allows for better data quality, facilitating subsequent analysis of clinical trial enrollment and trial outcomes. The participation rate will increase immediately, but we believe it will take 6-12 months to prove our enrollment goals for any given trial.
As more clinical trials are successfully completed utilizing our strategies, we hope the research community will learn of our approach and partner with Science 37 for future clinical trial work. More clinical trials completed via patient-centric will yield increased accrual rates over time. Also as awareness of the model increases, and media attention increases more patients will know they can search online for decentralized clinical trials. This will provide a positive feedback loop for recruitment.
Science 37 works closely with study sponsors, the FDA, Institutional Review Boards, healthcare lawyers and digital recruitment experts to assure that we are in compliance with all applicable federal and state regulations. That said, innovation is occasionally met with resistance. Our successful launch of a Phase III trial this month suggests we can be successful moving forward with cancer trials as well.
The technologies that allow for NTCs have only recently become accessible to a broad sector of the population. Our proposed approach is in current use for non-cancer related clinical trials. We are currently witnessing great successes in clinical trial recruitment. The creation of Science 37 -- an innovative and somewhat disruptive platform and service-based company -- required an incredibly complex set of skill sets and core competencies including expertise in clinical trials, telemedicine, digital recruitment strategies, and mobile technology developers. The recent advances in several of these domains means that the timing is perfect to launch the Science 37 platform.