CCC19 is collecting reports on adult patients with a current or historical invasive solid or hematologic malignancy who have been diagnosed with COVID-19, with or without laboratory confirmation of SARS-CoV-2 infection. Reporting is anonymous and all reported data must be de-identified. Data is collected through a REDCap Survey, by manual curation of electronic medical records. We share findings from registry rapidly through peer reviewed publications, followed by making aggregate data publicly available through R-Shiny Dashboard, hosted at the consortium website. The dashboard will be periodically updated with published datasets. Wherever needed, we mask fewer than five (5) cases.
Prompted by the need for rapid assessment of clinical impact of COVID-19 in patients with cancer, and to identify and share the best practices to facilitate care during this pandemic, an active conversation took place on Twitter and other social media platforms in March, 2020. The COVID-19 and Cancer Consortium (CCC19) was convened on March 15, 2020 by five founding institutions. The driving goal and mission statement of CCC19 is “to collect and disseminate prospective, granular, uniformly organized information on people with cancer who are diagnosed with COVID-19—at scale and as rapidly as possible". The participating sites identify and report data after obtaining necessary institutional IRB and data transfer approvals. The data is curated directly through REDCap web survey, or through files exported from local REDCap clones. The updated data dictionary of CCC19 is distributed through public Git hub repository. A Steering Committee selects pertinent research questions through consortium-wide application process. The Research and Data Coordination Centers facilitate the analytical and statistical rigor to draft the manuscript for the peer review submission and scientific presentations.
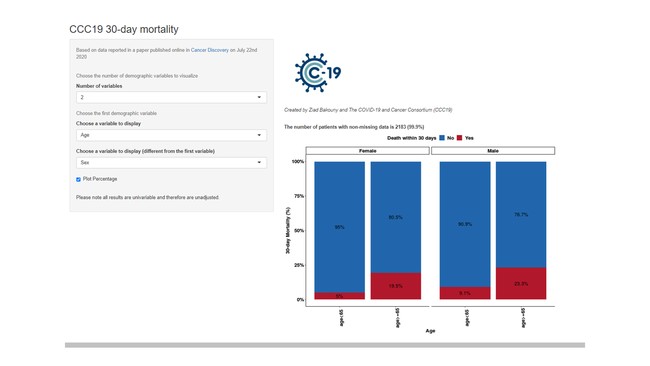
The CCC19 registry (www.ccc19.org; NCT04354701) is, to our knowledge, the largest curated registry of patients with cancer and COVID-19 in North America with >14,000 high-quality records from more than 80 contributing centers. Currently 126 institutions are actively participating in CCC19. Among the member institutions are 29 academic medical centers, 42 community practices, 10 NCI-designated Cancer Centers, 43 NCI-designated Comprehensive Cancer Centers, and 2 international Comprehensive Cancer Centers. The consortium is governed by a steering committee comprised of members with a diverse clinical and research background in oncology, hematology, viral epidemiology, clinical informatics, and biostatistics. Operational subcommittees include publications (to establish authorship guidelines for projects utilizing CCC19 data and/or resources), funding (to identify sources of funding for the consortium, disseminate this information to consortium members, and assist in the writing and critical revision of grants), epidemiology and biostatistics (to establish guidelines and provide support to investigators in designing and executing studies with the highest rigor, reproducibility, and impact), informatics (to develop and maintain the survey instrument[s] and oversee standardization of the data model, integration of data directly from EHRs, and visualization of data), and patient advocacy (to engage with cancer patient communities and advocacy networks and to coordinate with parties reaching out to CCC19). A large proportion of participating centers, 93 (74%), have made active contributions to the CCC19 registry accumulating N=14,120 case records with complete baseline forms; of which 8750 (62%) cases have at least 1 follow-up report, and the median duration of follow-up is 90 (IQR 30-180) days. This data has led to 12 peer reviewed published studies, accumulating 1522 citations. Our first publication in the Lancet hailed by the press as "From a Tweet to The Lancet in 10 Weeks", was an example of the remarkable efficiency of the crowdsourcing and volunteering. The Lancet publication was first to answer whether it was safe to treat cancer during the pandemic. Currently three more manuscripts are under review and more than six are in the advanced stage of preparation. In addition to these publications, investigators have also presented the CCC19 at regional, national, and international meetings.
Due to the challenges of temporality as well as the need to define composite outcomes and risk factors, a large number of derived variables have been developed. These have evolved in parallel with the main survey instruments. Given that many of the variables are optional and that there is an “Unknown” option for each variable, missingness and excessive unknown responses are concerns for both raw and derived variables. In order to mitigate these concerns, we developed a quality score that is used to evaluate case reports for targeted improvement. Data problems are classified as minor (1 point), moderate (3 points), and major (5 points). Quality score metrics are periodically returned to sites, and the overall change in the cumulative distribution of quality scores is shared with the consortium on a regular basis.
In our experience, dedicating ample resources towards rapid and open access distribution of critically important data is very important. Unfortunately, scientific publications are frequently behind paywalls, but CCC19 is very dedicated in making the publication publicly accessible. A very active Patient Advisory Committee further engages with the patient community to provide an easy to understand summary of the results.