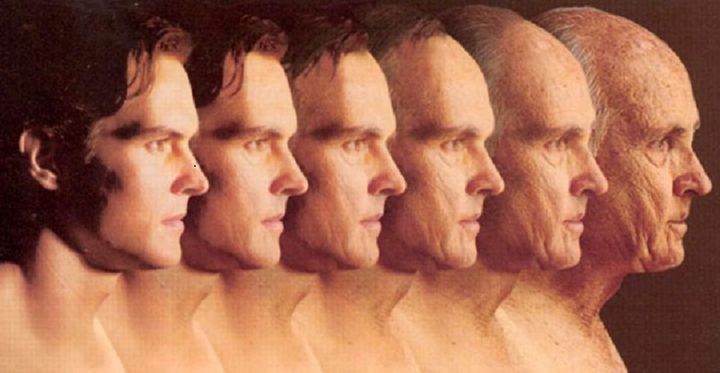
Clinical Immortality
It's what known as Clinical Immortality - the ability to artificially extend the lifespan of a living organism indefinitely. And in recent decades, between cryogenics, gene sequencing and biomedical research, there has been a great deal of speculation that such a thing may be possible someday soon. And this past January, another big step was taken in that direction.
Lengthening telomeres and extending lifespans
Researchers at Stanford University have developed a new procedure to lengthen telomeres in chromosomes. In so doing, they have effectively increased the number of times cells can divide, thus turning back the clock on the cell's aging process. This not only has useful applications for life-extension, but also for laboratory work and providing new means of treating various age-related disorders, heart disease and even muscular dystrophy.
In short, a telomere is a region of repetitive nucleotide sequences at each end of a chromatid. These "caps" are not only responsible for protecting the end of the chromosome from deterioration or from fusion with neighbouring chromosome, they are also associated with aging and age-related disease.
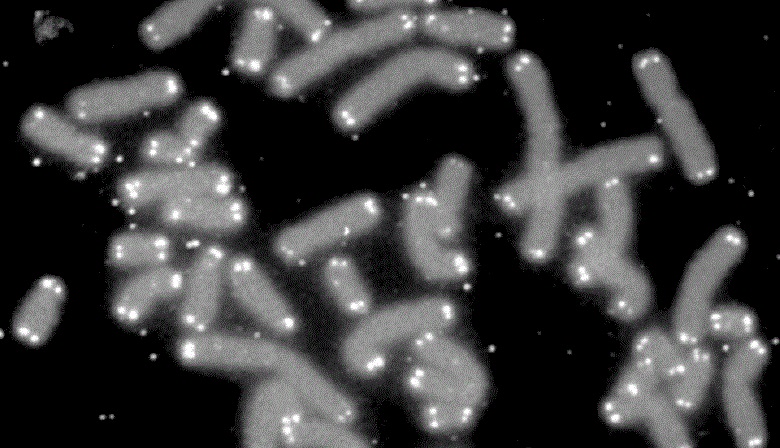
Microscopic view of human chromosomes (grey) capped by telomeres (white). Credit: U.S. DOE Human Genome Program
When a person is young, their telomeres are 8,000 to 10,000 nucleotides long, but this decreases with age. Every time a cell divides, the telomere's shorten, eventually reaching a critical length where they can no longer divide and will die. By lengthening a telomere by 1,000 nucleotides, the Stanford researchers were able to add many years to live of cells that underwent their process.
The procedure involved the use of a modified type of RNA, which in ordinary cell function acts as a sort of messenger, carrying carrying instructions from the DNA's genes to the cell's protein-making areas. In this case, the researchers inserted the active component telomerase (TERT), an enzyme that rebuilds telomeres and stem cells.
What the researchers found was, compared to the a culture of untreated cells, skin cells with lengthened telomeres were able to divide around 28 more times than untreated cells, while muscle cells divided about three more times. The researchers also found that as few as three applications of the modified RNA over a period of a few days could significantly increase the length of the telomeres in cultured human muscle and skin cells.
RNA acts as the DNA's messenger to the body's protein cells, instructing them to age. Credit: labgrab.com
But most important of all was the fact that the effects of the treatment were temporary, lasting only 48 hours before the newly lengthened telomeres began to progressively shorten again with each cell division. Since it was first discovered back 1980's, telomerase has been seen as a potential "fountain of youth". However, its indiscriminate use in cells could lead to uncontrolled cell growth, dramatically increasing the risk of cancer.
This is perhaps the most important and impressive aspect of the Stanford team's procedure, which is that it is temporary (and hence controllable). According to Dr. Helen Blau - a professor of microbiology and immunology at Stanford and director of the university’s Baxter Laboratory for Stem Cell Biology - this discovery has multiple benefits:
“Now we have found a way to lengthen human telomeres by as much as 1,000 nucleotides," she said, "turning back the internal clock in these cells by the equivalent of many years of human life. This greatly increases the number of cells available for studies such as drug testing or disease modeling.”
Treating and preventing diseases with lengthened telomeres
A 1,000-nucleotide addition represents a more than 10 percent increase in the length of the telomeres. Given that the average global life expectancy is 67.2, this could also mean a life extension of about 7 years. The Stanford team and other researchers are now testing the technique on specific types of muscle cells, looking for ways to treat Duchenne muscular dystrophy and heart disease.

Salk researchers Victoria Lundblad and Timothy Tucey. Credit: Salk Institute for Biological Studies
But of course, the Standford procedure does is merely the latest in a long line of breakthroughs involving telomere and telomerase research. For example, in September of 2014, Victoria Lundbald and Timothy Tucey - two researchers at the Salk Institute - announced the discovery of an "on-and-off switch” in cells that may hold the key to healthy aging.
Much like the Stanford process, the Salk researchers introduced the telomerase enzyme to telomeres in order to extend their capacity for cell division. However, their research went beyond this to discern that the formation of active component telomerase also gives rise to the formation of an inactive counterpart, a disassembly pathway that may provide a means of keeping telomerase at exceptionally low levels inside the cell.
By being able to regulate the presence of telomerase in cells, it could be possible to both control aging while preventing the formation of certain types of cancer.
And then there was the research conducted in 2010 at the Dana-Farber Cancer Institute and Harvard Medical School in Boston, Massachusetts. Here, researchers found that the aging process in mice could be sped up by removing telomerase, and reversed by reintroducing it into their cells. Their research appeared in the November 28th, 2010 issue of Nature.
Aging, once thought to be an inavoidable scourge that humans simply had to deal with, may finally have met its match! And while a considerable amount of research still needs to be done before telomere-lengthening or telomerase treatments could be considered a regular part of medical science, the possibilities are certainly intriguing, and perhaps just the slightest bit frightening.
An age of post-mortality... Who amongst us would not feel both excitement and trepidation at such a possibility?
Top image: bodybuilding.elitefitness.com
Sources:
- www.gizmag.com/telomere-extension-aging/35816/
- www.news-medical.net/health/Telomere-Lengthening.aspx
- www.fasebj.org/content/early/2015/01/21/fj.14-259531.abstract
- med.stanford.edu/news/all-news/2015/01/telomere-extension-turns-back-aging-clock-in-cultured-cells.html
- www.gizmag.com/on-off-switch-telomerase-aging-salk-institute/33931/
- www.salk.edu/news/pressrelease_details.php?press_id=2052
- genesdev.cshlp.org/content/early/2014/09/18/gad.246256.114.abstract
- www.nature.com/news/2010/101128/full/news.2010.635.html