1. Geographical and organizational barriers:
The greater Barcelona area holds over 4 million inhabitants and approximately 35.000 new cases of cancer are diagnosed every year. Cancer patients treated in the public Health Care System are administratively allocated to tertiary hospitals according to their street address (zip code). This fact significantly restricts the patient’s access to clinical trials run elsewhere since there are no mechanisms to overcome these geographical and administrative hurdles. Therefore, in the absence of an organized patient referral system, one given patient may only be exposed to the clinical trials that are ongoing in his designated hospital within the neighbourhood where he lives.
2. Low awareness and non-existing match-making tools:
Both physicians and patients lack awareness about cancer clinical trials conducted in the public Health Care System. There is not any “integrated cancer trials information centre”, and there is lack of systems that could match recruitment needs with the needs and preferences of cancer patients (and these of their families).
3. Lack of clear processes and standardization:
The clinical management practices of cancer patients across the different hospitals of greater Barcelona are not standardized, resulting in limited patient access to clinical trials and suboptimal genomics-based clinical research efficiency .
Link 1 (BARRIER 1) Description: Geographic barriers preclude access of patients to clinical trials.
Link 2 (BARRIER 2) Description: Reference sites do not exist for patients and their families to get informed and tracked.
Link 3 (BARRIER 3) Description: Clinical trials loose potential patients due to administrative/economic barriers among clinical sites.
BCTP IMPLEMENTS A COMPETITIVE CANCER CLINICAL MANAGEMENT SYSTEM THAT INTEGRATES ONCOLOGY TRIALS INTO THE STANDARD CLINICAL CARE DECISSION SYSTEMS
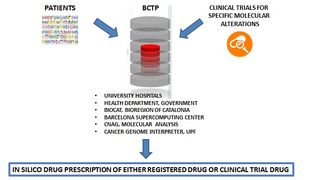
BCTP brings together seven university hospitals that care for over 4 million people. BCTP encompasses the implementation of highly effective genomics-based clinical decision support systems to personalize therapies according to the patient´s profile, thus optimizing not only actual patient outcomes but also the speed and effectiveness of the whole clinical development pathway. BCTP places the patient at the centre while it gathers the necessary commitment from the scientific and medical partners, the political support from the Health Department and, the managerial skills to successfully work across the geographical and administrative barriers.
BCTP EXPECTS TO TRIPLICATE THE RECRUITMENT RATE IN ONCOLOGY CLINICAL TRIALS
The Barcelona area is by far the region of Spain with a strongest tradition in clinical trials. More than 50% of the clinical trials run in Spain have been reviewed by an IRB located in Barcelona. However, the Regional Health Department data show that even though every year 35.000 new cancer cases are diagnosed in the area, only approximately 1/10 is eventually enrolled in a clinical trial. The reasons for this low recruitment rate have been described above. With the BCTP solution we expect this ratio to grow three fold (this is that we expect, in the future, to include 3 of every 10 newly diagnosed patients in an oncology clinical trial).
Please note that at this stage (BCTP ideation phase) the specific increase in recruitment rate is unknown. One of the purposes of GENPRECIS.CAT pilot run (BCTP implementation phase) is to measure the variation in patient accrual rates.
BCTP PROPOSES A STRUCTURAL CHANGE IN THE WAY OF CONDUCTING ONCOLOGY TRIALS THAT MAY BE EXTENDED TO OTHER REGIONS
BCTP represents a long term structural change in the way of conducting cancer clinical trials in Barcelona area. It has the explicit support of the Health Department as well as the commitment of the University Hospitals and genomics platforms involved. We anticipate that the impact of BCTP, as measured by clinical outcomes and speed and quality of cancer clinical trials will improve in the next 2-3 years, to become one of the leading hubs in cancer clinical trial performance in Europe. As next steps, this model can be applied to other indications and can also be implemented in other regions, thus sustaining growth of patient accrual rates.
BIOCAT PROVIDES STRONG LEADERSHIP AS WELL AS MANAGERIAL AND PROJECT MANAGEMENT SKILLS TO ENSURE SMOOTH AND EFFICIENT PROCESSES IN AN OPTIMAL LEGAL AND REGULATORY FRAMEWORK
BCTP is based on formal agreements among Biocat (Bioregion of Catalonia) and the 7 largest University Hospitals of the area with the support of the local Government. to dynamize all the stakeholders in this area and their initiatives in order to create an environment with a strong research system, active transfer of knowledge and an entrepreneuring business fabric that can become a driving force for the country’s economy and contribute to the wellbeing of society as a whole.
THE RIGHT PARTNERS COME TOGETHER FOR THE FIRST TIME IN THE BARCELONA AREA
Even though there are successful experiences of similar models in other territories, particularly in the NHS in UK, this is the first time that Catalonia has got the right leadership at Biocat and the commitment of the Catalan Government at its highest level to implement a competitive clinical trials platform at a large scale. These two conditions have made it possible to coordinate not only the 7 largest University Hospitals of Catalonia but also a number of highly competitive cancer research centres and genomics facilities to effectively run personalized clinical trials (e.g.: the logistics of sampling and sequencing has been optimized to a point where the cost per patient is affordable at a whole population level).