Clinical trials are critical to advancing health, saving millions of lives by delivering state-of-the-art programs in screening, detection, and treatment, providing the means to rigorously evaluate cutting edge science, and forging a path toward a cure for many of the most devastating illnesses, including cancer.
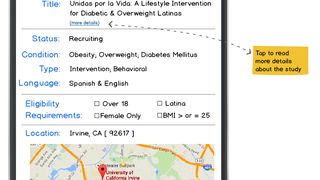
ClinicalTrials.gov, the registry and results database supported by the National Institutes of Health, currently lists 189,473 studies with locations in all 50 states and in 190 countries. However, low patient participation in the U.S. is widespread despite the benefits and opportunities afforded by clinical trial participation. Lack of awareness is the largest contributing factor (85%) to low participation (Crookes et al., 2015). Other factors include perceptions of geographic inaccessibility, language barriers, and mistrust (Byrne et al., 2014). Of particular concern is the low participation of minority and low socioeconomic (SES) populations (Ghebre et al., 2014). All of these barriers point to a lack of easily accessible, comprehensible, and usable information about clinical trials among cancer patients.